Orphan Drug Designation Consulting
Accelerate FDA & EMA orphan drug designation + market authorization with the industry's most trusted regulatory experts.
Dr. Timothy Cote
-CEO, Only Orphans Cote
Rare Diseases and Orphan Drugs Focus
Our team is ready to win for you. From data assessment to FDA/EMA submissions we have the expertise you need to get your application approved. Rare diseases deserve attention. Our unmatched regulatory services make all the difference.
REGULATORY SERVICES AND STRATEGIES
OUR ORPHAN DRUG CONSULTING COMMITMENT
At Only Orphans Cote, you gain the advantage of working directly with Dr. Timothy Cote, former Director of the FDA’s Office of Orphan Product Development. His first-hand regulatory insight and decades of experience give you the inside track to navigate orphan drug designation with clarity, speed, and confidence.
While at the helm of the FDA's OOPD, Dr. Cote personally decided on 1400 orphan designation applications and saw 200 orphan drugs go to market. His first consultancy (2012-2017) serviced about 500 clients before its sale to IQVIA. Since July 2021, this new consultancy has spurred an additional 120 clients on orphan drug regulatory matters. As both a public servant and industry consultant, Dr. Cote has unique and substantive expertise with regulatory work in orphan drugs. Let his experience work for you.

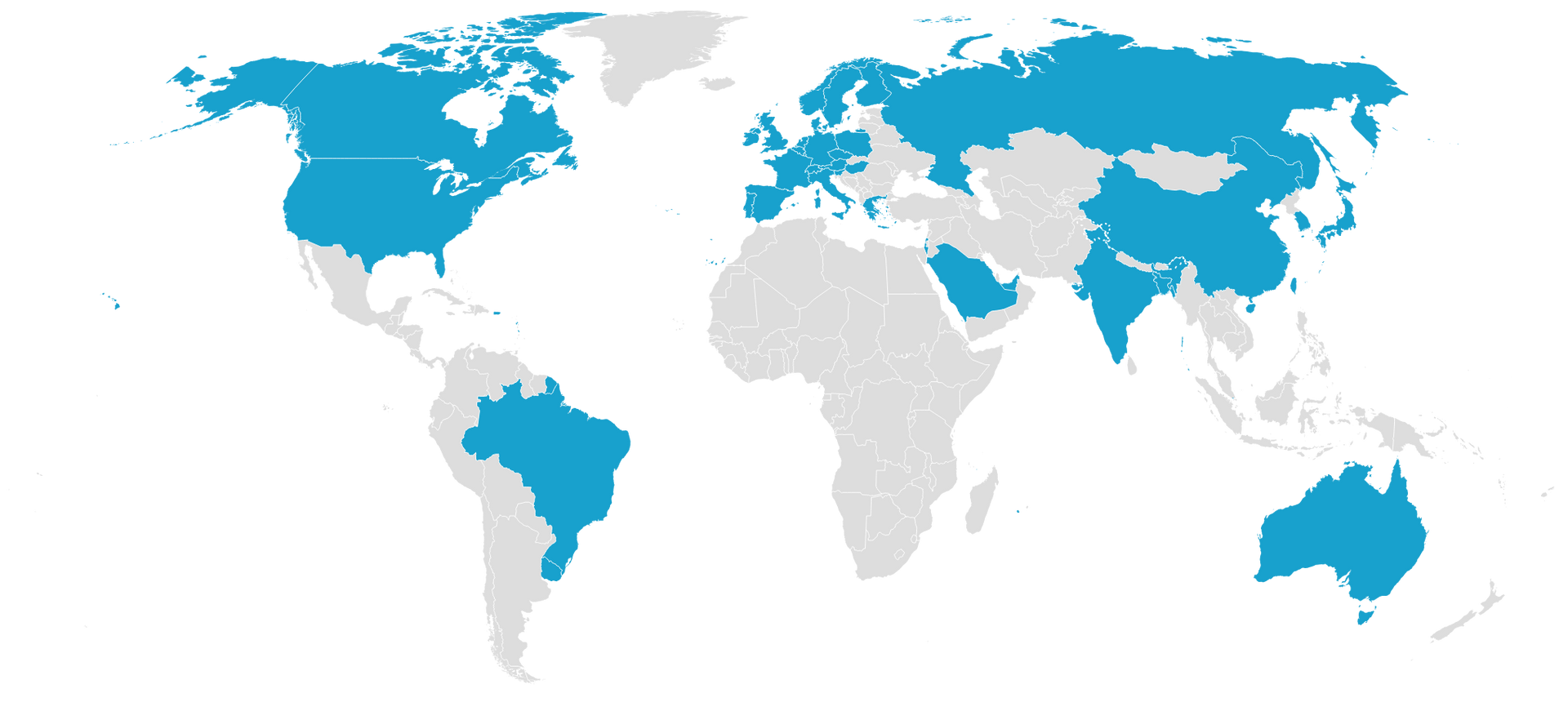
OUR REGULATORY AFFAIRS GLOBAL IMPACT
Together with our clients in North America, Europe, Asia and the Middle East, we are impacting the orphan drug market.
Meet the OOC Team at Upcoming Biotech Conferences and Events 2025
World Orphan Drug Congress 2025
April 22-24 Boston, MA
LSX World Congress USA
September 16-17 Boston, MA
ASGCT 2025
May 13-17 New Orleans, LA
RAPS Convergence
October 7-9 Pittsburgh, PA
BIO International 2025
June 16-19 Boston, MA
Bio Japan 2025
October 8-19 Yokohama, Japan
Medical Rationale Criteria
What is needed for orphan drug designation? Meeting the Medical Rationale Criteria is key! Dr. Cote breaks down the benchmark elements
Prevalence criteria
The Prevalence Criteria is a quantitative story. There is no list, you have to make it up! Dr. Cote tells you how to do so accurately in this orphan minute.
PRV
How can you be eligible for a PRV? Dr. Cote is here for your orphan minute to give you the inside scoop on how producing Medical Countermeasures are one way that lead to the PRV.
Orphan Exclusivity
What is orphan exclusivity? What are the guidelines for it? Dr. Cote details the rules and structure in this orphan minute.




